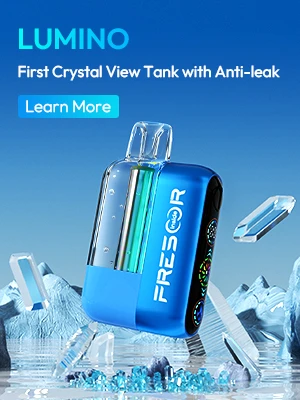
On June 14, the FDA released the following picture on its official website, which roughly means: More and more e-cigarette manufacturers are using synthetic nicotine to manufacture products to evade FDA supervision. But an important federal law that went into effect in April clarifies that the FDA has the power to regulate tobacco products containing nicotine from any source, including synthetic nicotine, so the FDA will continue to enforce regulation of synthetic nicotine products.
The FDA has issued its first two warning letters today to manufacturers for illegally selling synthetic nicotine products without authorization. Together, the two companies, AZ Swagg Sauce LLC and Electric Smoke Vapor House, have about 10,000 products listed with the FDA. As required by the new law, neither company has submitted a premarket application for its non-tobacco nicotine product by the May 14, 2022 deadline.
At the same time, the FDA also announced that it has issued 107 warning letters to retailers in the past two weeks for illegally selling synthetic nicotine products, including certain e-cigarettes or e-liquids, to underage buyers. And new federal law prohibits the sale of tobacco products (including synthetic nicotine products) to customers under the age of 21

Current progress: It has directly affected the export of disposable vapes in China
In this news, the FDA also reiterated the regulatory approach to synthetic nicotine products and the latest progress of the work:
From July 13, 2022, any synthetic nicotine products that have not passed the PMTA cannot be legally marketed. The FDA is currently processing approximately 1 million applications for synthetic nicotine products submitted by more than 200 manufacturers by the May 14, 2022 deadline. The FDA is preparing to issue rejection letters as soon as possible for those applications that do not meet the criteria.
FDA is doing the necessary work to take enforcement action as quickly as possible: When illegal sales of non-tobacco nicotine products are detected, FDA typically first issues a warning letter to drive proactive compliance and decides whether to take enforcement action (including civil fines, bans, etc.) as needed order, seizure or injunction). Additionally, any unauthorized synthetic nicotine products detected for import into the United States may be detained or denied entry.
To summarize the above information, synthetic nicotine products can no longer be freed from FDA supervision, and synthetic nicotine products in the US market are about to be removed from the shelves. And those cargo ships full of synthetic nicotine products bound for the United States are also very likely to be unable to dock due to this.
We also checked with relevant practitioners and learned that the FDA’s move has indeed been passed to the upstream. According to practitioners, the U.S. Customs has begun to seize a large proportion of the goods, and they need to wait for the FDA to inspect the goods before they can be released. At the same time, because the FDA requires the terminal to be rectified and removed from the shelves, many domestic manufacturers have made a lot of goods. We can only wait for American customers to pick up the goods; and those who have already picked up the goods have not paid due to poor local sales.
According to practitioners, as far as he knows, there are other practitioners who have tens of millions of payment for goods that have been unable to recover, or have made tens of millions of goods that are not waiting for customers to pick up the goods. This practitioner lamented to us that “it is a time bomb at one time, and the risk is high; there are still a group of people who come in and dream of getting rich.”
Context of development: Regulatory synthetic nicotine is not without precursors
It needs to be clear, however, that the core of the relevant federal laws and the various actions of the FDA is not to “drive synthetic nicotine products out of the United States”, but to “incorporate synthetic nicotine into regulatory supervision”. So the question is, how is synthetic nicotine free from FDA supervision?
FDA regulation of tobacco products dates back to 2009. Then-President Barack Obama signed the Family Smoking Prevention and Tobacco Control Act, which defined a tobacco product as “any product made or derived from tobacco for human consumption.” Since then, both traditional tobacco and e-cigarettes have been regulated by the FDA.
In 2020, the FDA began to promote the ban on flavored e-cigarettes other than tobacco and menthol. This means that disposable products that are popular in various flavors in the United States are facing a complete delisting. For example, Puff Bar, the most well-known e-cigarette brand in the U.S. market (and the one that has the greatest impact on teenagers), received a notice from the FDA in July 2020, requiring it to stop selling flavored products. But just a few months later, in March 2021, Puff Bar returned to the market with synthetic nicotine.
This is undoubtedly a loophole in regulation. Based on relevant laws, the FDA’s regulatory scope is only tobacco and electronic cigarettes that use naturally extracted nicotine. The synthetic nicotine used by the disposables represented by Puff Bar is not extracted from tobacco, and naturally claims not to be controlled by the FDA. As a result, a large number of flavored e-cigarettes have made a comeback in a one-off form. According to data from a Stanford University paper, there are at least six synthetic nicotine manufacturers participating in the U.S. market, and 98 brands that claim to use synthetic nicotine.
And a 2021 investigation by the FDA and CDC shows. Puff Bar, which has a variety of flavors, is the most popular brand among middle school and student e-cigarette users, with as many as 26.8% saying that Puff Bar is their “common brand”, while Juul, which has been restricted by flavor bans, ranks fourth. , only 6.8%.
This way of evading supervision has naturally attracted the attention of American society. All parties began to urge the FDA to solve the problem of the one-time overflow of flavorings as soon as possible. So in March of this year, the relevant bill was officially passed and signed by Biden, which clearly brought synthetic nicotine products into the FDA’s regulatory scope. And make it clear: All e-cigarette companies that use synthetic nicotine must apply for pre-market authorization by May 14, and all existing products that have not been authorized must be removed from the market by July 13.
The above is the antecedent of this “off-the-shelf storm”.
Roots: Why regulation of synthetic nicotine is suddenly in full swing
In fact, after this new regulation was passed in March. Some relevant practitioners are optimistic about the continued sales of synthetic nicotine products.
A U.S. lawyer specializing in tobacco law said in April that companies that use synthetic nicotine and the FDA are unlikely to meet the deadline set by Congress because of the heavy workload of filing and reviewing applications. And Stacy Ehrlich, a partner at Kleinfeld Kaplan & Becker LLP of Tobacco Manufacturing Group Consulting, said PMTA applications “usually require multiple studies, almost all of which last more than 60 days”.
“If they don’t start processing their” application before this legislation is enacted, it will be very difficult to complete a satisfactory “application” by May 14, she said. Lawyers speculate that a July deadline After that, the FDA may not take enforcement action against the pending applications, essentially allowing them to remain on the market. Because the FDA has not strictly enforced the previously set deadline of September 2021, a large number of products that have not passed PMTA are still on sale.
But the FDA may be starting to play the real game this time, after all, it is under more pressure.
A month ago, for example, more than 30 U.S. attorneys general formed a coalition calling on the FDA to reject all applications for marketing authorization for synthetic nicotine products. They believe that synthetic nicotine products do not meet the FDA’s public health standards, and the effects of synthetic nicotine on the human body are not yet clear. They also mentioned the “unfairness” in the regulation of synthetic nicotine products versus extracted nicotine products—synthetic nicotine products escaped regulation, while extracting nicotine products had to spend expensive efforts to comply with FDA standards.
According to foreign media reports, the FDA is facing pressure from lawmakers to implement the new regulations. Senators Sens. Dick Durbin (D-Ill.) and Susan Collins (R-Maine) previously helped push the FDA’s new regulatory agency in Congress, health news outlet STAT reported on July 13. In a recent letter to FDA Commissioner Robert Califf, they clearly mentioned that the FDA “failed to take enforcement action against manufacturers of electronic cigarettes that are illegally placed on the market.”
Senator Durbin, in particular, believes the FDA commissioner should resign if he can’t review these products sooner, health news outlet STAT reported. In fact, the FDA’s recent move to ban Juul has drawn similar comments from lawmakers — a move that Durbin says is a sign of the FDA’s “incompetence.”
But at the same time, the letter from the two senators also pointed the way to the FDA: The FDA can “correct the error”: Starting Wednesday, the FDA can legally “remove from the market all unauthorized e-cigarettes that use synthetic nicotine”, and They asked the FDA to respond by July 20 to a series of questions about compliance, such as how many synthetic nicotine companies have not filed on time and how many warning letters the FDA has issued.
In other words, Congress has issued an ultimatum to the FDA, and it is even directly related to the FDA director’s black gauze cap. It is not difficult to understand such a vigorous enforcement of relevant regulations. Although the focus this time is on one-time synthetic nicotine, because the relevant supervision has been further strengthened, this is undoubtedly a greater test for companies involved in the US market.